Biotechnology-derived products (BDPs), such as monoclonal antibodies, gene therapies, and recombinant proteins, are a growing part of modern medicine. These products hold great promise for treating diseases, but they also come with strict regulatory
Holiday Staffing Gaps and Biosafety Waste: Minimizing Risk During Low-Supervision Periods
The holiday season often means fewer people on-site, shorter hours, and less direct supervision in labs, clinics, and healthcare facilities. While many operations slow down, biosafety waste management cannot be paused. Improper handling or delayed di
Safety Responsibilities for IRB Members: Protecting Both Subjects and Staff
Institutional Review Boards (IRBs) play a key role in research oversight. Their job goes far beyond reviewing study designs and consent forms. IRB members are responsible for protecting the rights, safety, and well-being of human subjects. But anothe
Quick Tips for Complying with In Vivo Bioequivalence Program Requirements
Conducting in vivo bioequivalence (BE) studies is a critical step for companies developing generic drugs. These studies compare a test product to a reference drug to show that both perform the same in the human body. The U.S. Food and Drug Administra
How to Prepare for an FDA BIMO Inspection: A Clinical Investigator’s Compliance Guide
Preparing for an FDA BIMO Inspection When the FDA notifies you of a Bioresearch Monitoring (BIMO) inspection, it can feel overwhelming at first. These inspections are designed to review the quality and
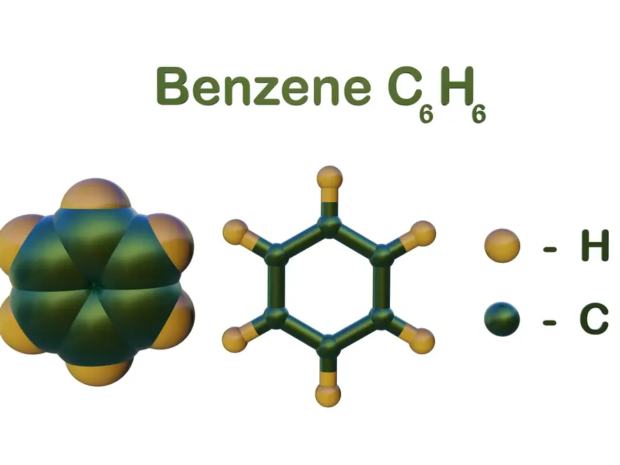
Engineering Controls for Benzene Exposure: What the Data Says About Their Effectiveness
Got it! From now on, I’ll bold all statistical data in your articles as per your preference. Let me now continue and complete the article with that in mind:Engineering Controls for Benzene Exposure: What the Data Says About Their EffectivenessBenzene
OSHA Requirements for Bench and Pedestal Grinders: What the Law Demands
OSHA Grinder Safety - Bench & Pedestal Introduction Bench and pedestal grinders are common tools in many workshops and maintenance a
Battery and Charger Safety During Holiday Downtime: Prevent Fires and Leaks
Holiday season means winding down operations, but batteries and chargers still require attention—even when staff are away or shifts are reduced. Whether your facility uses forklifts, backup power systems, or everyday electronics, risks don’t pause ju
Documentation Safety: Why Accurate Batch Records Protect More Than Just Compliance
Every batch of pharmaceutical product starts with documentation. From raw material receipts to production logs, each step in manufacturing is recorded in batch records. On paper, this may look like a mundane task. In reality, those pages are vital to
What You Can (and Can’t) Give Healthcare Professionals Under PhRMA
Pharmaceutical companies and ethical rules PhRMA / Compliance Pharmaceutical companies often interact with healthcare professionals to share information about drugs, but e