Worker Safety • Environmental Awareness Practical tips that support safety, improve environmental performance, and align with OSHA/EPA guidance. Online Training Resource Read time: 6–8 minutes Table of Contents Introduc
Lockout/Tagout Safety Tips to Prevent Workplace Injuries
Practical tips to protect workers from hazardous energy and support OSHA compliance. Safety Guide OSHA 29 CFR 1910.147 · Subpart S Table of Contents Introduction OSHA Context: Why Lockout/Tagout Matters Common Electrical
Pharmacovigilance Reporting Explained: From Expedited to Aggregate Reports
Pharmacovigilance (PV) is the science and practice of monitoring drug safety. It focuses on identifying, assessing, and preventing adverse drug reactions (ADRs) and other drug-related problems. A key part of PV is reporting. Reports are how informati
Holiday Traffic Jams: Avoiding Tailgating in Congested Travel Seasons
The holiday season in the U.S. is one of the busiest travel times of the year. Thanksgiving, Christmas, and New Year’s bring millions of drivers onto highways and local roads. With the extra traffic comes a higher risk of collisions, many of them lin
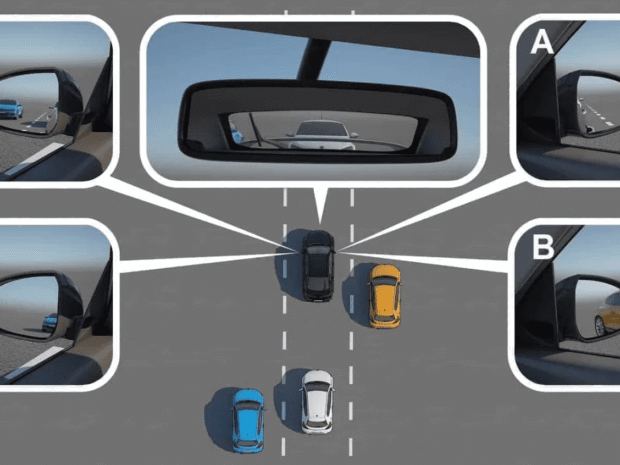
5 Lane-Changing Tips Every U.S. Driver Should Know
Changing lanes is one of the most common driving maneuvers, yet it is also one of the riskiest. A simple mistake during a lane change can cause collisions, road rage, or traffic delays. Every driver in the U.S. faces this situation daily, whether on
The Most Common Validation Documentation Mistakes (and How to Avoid Them)
Validation is a critical part of regulated industries like pharmaceuticals, medical devices, and dietary supplements. It proves that processes, equipment, and systems work as intended and meet compliance requirements. However, one of the biggest c
The Most Overlooked cGMP Rule for Supplement Facilities (and How to Fix It)
If you run or manage a dietary supplement facility, you're probably aware of FDA’s current Good Manufacturing Practices (cGMPs) under 21 CFR Part 111. You may have protocols for sanitation, batch records, supplier qualification, and equipment checks.
8 Design Control Tips Every Medical Device Team Should Follow
Design controls help medical device teams create products that are safe, effective, and meet regulatory rules. From concept to launch, following good design practices can save time, cut costs, and prevent recalls. This guide highlights eight key tips
How Quality Assurance Supports Data Integrity from Start to Finish
In clinical research, accurate and trustworthy data is the foundation for every decision, from trial results to regulatory approvals. But this level of trust doesn’t happen by chance. Quality Assurance (QA) helps protect data integrity at every step.
How to Use Hand Signals That Every Crane Operator Understands
Crane Hand Signals: A Guide for Safer Construction Sites On busy construction sites, clear communication can be the difference between safe lifting and serious accidents. When noise levels are high or radios fail, hand signals become the main lan